CUSTICS SINGAPORE PTE. LTD. has announced that its product, Hautuki, has already completed registration with the U.S. FDA Administration OTC Product.
Maharastra, India, 24th August 2024, According to a company representative, Hautuki was developed by CUSTICS Co., Ltd. using TDDS (Transdermal Drug Delivery System) technology previously acquired from Seoul National University. This innovative patch integrates vitamins and various nutrients and is specifically designed to support children’s growth.

The technology is the “Pore-Permeable Transdermal Peptide Ligand,” a patented innovation from the Department of Bio engineering at Seoul National University. This technology is particularly useful for facilitating the delivery of large active ingredients through the skin, enhancing the efficacy of transdermal administration.
A representative from Hautuki stated, “In addition to the FDA OTC registration, we are pursuing other certifications as well. We advise caution when purchasing from sources other than our official store, as products from unofficial channels may be counterfeit.”
They added, “We will continue to work diligently to ensure that Hautuki consumers can purchase without doubt.
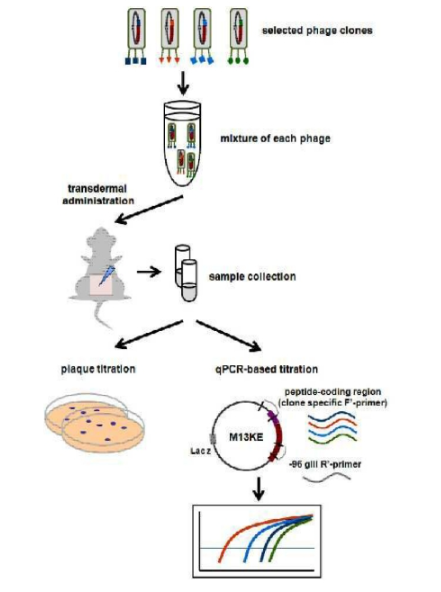
Diagram of a transdermal delivery peptide that penetrates the skin through pores, originally filed by Seoul National University Industry Foundation on March 11, 2011, and transferred to Custics Co., Ltd. on February 8, 2021.
Official mall : Hautuki Singapore
Patent Information : Hautuki’s Patent Technology
Distributor Name – Amit Biswas
Distribution Agency Name – Grow With Amit
Distributor Contact Number – 1169312053


Comments